Background. The only curative treatment of Myelodysplastic syndromes (MDS) is represented by allogeneic hematopoietic cell transplantation (HCT). The risk scoring system conventionally used in clinical practice for transplant decision-making is IPSS-R (Greenberg PL et al, 2012), which has been shown to predict the HCT outcome(Della Porta MG et al, 2014). The recent broader application of next generation sequencing (NGS) in clinical practice provides an abundance of molecular data and poses the challenge of better prognostic stratification in MDS patients. Recently, Bernard et al(Elsa Bernard et al, 2022) developed a molecular prognostic score called IPSS-Molecular (IPSS-M) which defines six risk categories based on the patient's molecular profile combined with clinical and cytogenetic data. In the original study, only 9% of patients underwent HSCT, therefore the utility of this score in the transplant decision making needs to be explored.
Aim.The aim of this study is to evaluate the impact of IPSS-M on the outcome of HCT in patients with MDS.
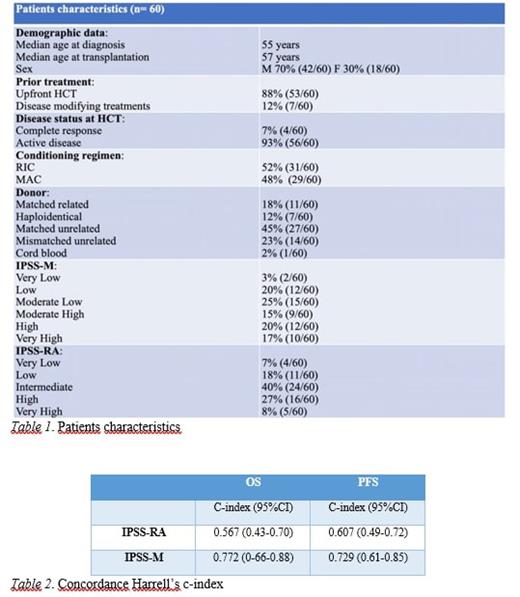
Methods and Results. This is a single center study including 60 consecutive MDS patients who received allogeneic HCT from 2004 to 2023 at Papa Giovanni XXIII Hospital, Bergamo. The median age at transplantation was 57 years and 70% were males. HCT related parameters are listed in Table 1. Of note, 53 out of 60 patients (88%) received an upfront HCT. According to IPSS-RA (age-adjusted IPSS-R), patients clustered in Very Low (4/60), Low (11/60), Intermediate (24/60), High (16/60) and Very High (5/60) risk groups. We retrospectively performed a NGS study before HCT by using a panel of 30 genes for all patients who had available DNA and calculated IPSS-M score for each patient. According to IPSS-M, patients clustered in Very Low (2/60), Low (12/60), Moderate Low (15/60), Moderate High (9/60), High (12/60) and Very High (10/60). Re-stratification occurred in 59% of patients (35/60), of which 22% (13/60) were down-staged and 37% (22/60) were upstaged. With a median follow-up time of 7 years after HSCT, the 5-year overall (OS) and relapse-free survival (RFS) was 67% and 64% respectively. In order to evaluate the utility of IPSS-M in estimating OS and RFS in HCT setting compared to IPSS-R, a concordance test was performed. In terms of OS and RFS, IPSS-M resulted respectively in a C-index of 0.772 and 0.729 compared to 0.567 and 0.607 of IPSS-R (table 2).
Conclusions. Despite the limitations due to the small sample and the retrospective design of the study, we conclude that IPSS-M performed better compared to the conventional score in predicting RFS and OS of our MDS patients undergoing HSCT, providing data supporting the integration of clinical and molecular information in this setting. Further improvements may be achieved by integrating the molecular data with clinical information regarding HSCT-related parameters such as performance status, donor and conditioning regimen.
Disclosures
Frigeni:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Honoraria. Lussana:Clinigen: Membership on an entity's Board of Directors or advisory committees; Amgen: Speakers Bureau; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Membership on an entity's Board of Directors or advisory committees. Rambaldi:Abbvie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal